Na mgbakwunye na nkà na ụzụ, njikọ nke glycosides na-enwe mmasị mgbe nile na sayensị, n'ihi na ọ bụ mmeghachi omume na-emekarị na okike. Akwụkwọ ndị Schmidt na Toshima na Tatsuta dere n'oge na-adịbeghị anya, yana ọtụtụ nrụtụ aka ndị e zoro aka na ha n'ime ya, ekwuwo okwu n'ụdị dị iche iche nke ike sịntetik.
Na njikọ nke glycosides, a na-ejikọta ihe mejupụtara shuga dị iche iche na nucleophiles, dị ka alcohols, carbohydrates, ma ọ bụ protein, ma ọ bụrụ na mmeghachi omume nhọrọ na otu n'ime otu hydroxyl nke carbohydrate chọrọ, a ga-echekwa ọrụ ndị ọzọ niile na nzọụkwụ mbụ. Na ụkpụrụ, usoro enzymatic ma ọ bụ microbial, n'ihi nhọrọ ha, nwere ike dochie nchebe kemịkalụ dị mgbagwoju anya na usoro mgbochi iji họrọ site na glycosides na mpaghara. Otú ọ dị, n'ihi ogologo akụkọ ihe mere eme nke alkyl glycosides, ntinye nke enzymes na njikọ nke glycosides amụbeghị ma tinye ya n'ọrụ.
N'ihi ikike nke usoro enzyme kwesịrị ekwesị na ọnụ ahịa mmepụta dị elu, njikọ enzymatic nke alkyl polyglycosides adịghị njikere ịkwalite ọkwa mmepụta ihe, na usoro kemịkalụ na-ahọrọ.
N'afọ 1870, MAcolley kọrọ njikọ nke "acetochlorhydrose" (1, figure2) site na mmeghachi omume nke dextrose (glucose) na acetyl chloride, nke mechara mee ka akụkọ ihe mere eme nke ụzọ njikọ glycoside.
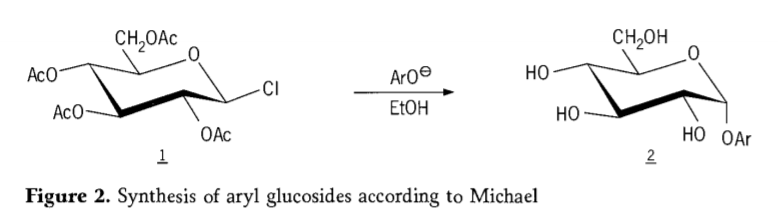
Achọpụtara Tetra-0-acetyl-glucopyranosyl halides (acetohaloglucoses) dị ka ihe etiti bara uru maka njikọ stereoselective nke alkyl glucosides dị ọcha. N'afọ 1879, Arthur Michael nwere ihe ịga nke ọma n'ịkwado aryl glycosides nke dị na Colley's intermediates na phenolates. (Aro-, foto 2).
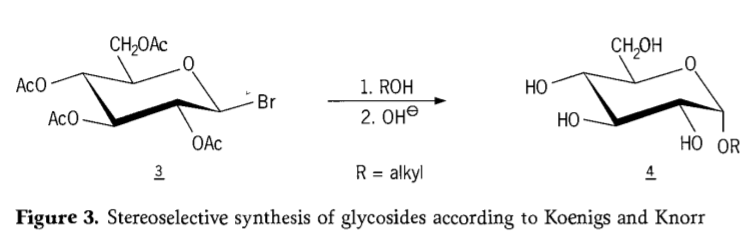
Na 1901, Michael's njikọ na a sara mbara nso nke carbohydrates na hydroxylic aglycons, mgbe W.Koenigs na E.Knorr webatara ha mma stereoselective glycosidation usoro (Nyocha 3). Mmeghachi omume na-agụnye ngbanwe SN2 na carbon anomeric ma na-enweta stereoselectively na ntụgharị nke nhazi, na-emepụta dịka ọmụmaatụ α-glucoside 4 site na β-anomer nke aceobromoglucose intermediate 3. Njikọ Koenigs-Knorr na-ewere ọnọdụ n'ihu ndị na-akwalite ọlaọcha ma ọ bụ mercury.
N'afọ 1893, Emil Fischer tụpụtara ụzọ dị iche iche maka njikọ nke alkyl glucosides. A maara usoro a ugbu a nke ọma dị ka "Fischer glycosidation" ma na-agụnye mmeghachi omume acid-catalyzed nke glycos na alcohols. Ihe ndekọ akụkọ ihe mere eme ọ bụla kwesịrị ịgụnye mbọ mbụ A.Gautier mere na 1874, ịtụgharị dextrose na ethanol anhydrous n'ihu hydrochloric acid. N'ihi nyocha nke elementrị na-eduhie eduhie, Gautier kwenyere na o nwetara "diglucose". Fischer mechara gosi na Gautier's “diglucose” bụ n'ezie ethyl glucoside (Fig 4).
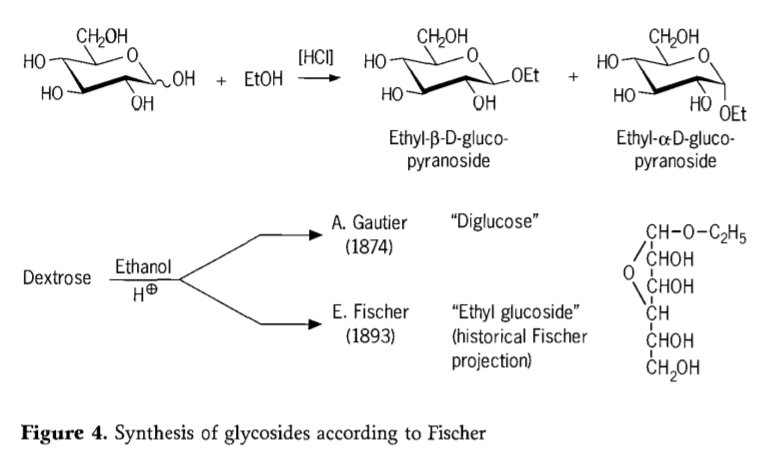
Fischer kọwapụtara nhazi nke ethyl glucoside nke ọma, dịka enwere ike ịhụ site na usoro furanosidic nke akụkọ ihe mere eme nke a tụrụ aro. N'ezie, ngwaahịa Fischer glycosidation dị mgbagwoju anya, na-abụkarị ngwakọta nha nke α / β-anomers na pyranoside / furanoside isomers nke nwekwara njikọ glycoside oligomers na-enweghị usoro.
N'ihi ya, ụdị molekụla nke ọ bụla adịghị mfe ikewapụ na ngwakọta mmeghachi omume Fischer, bụ nke bụ nsogbu siri ike n'oge gara aga. Mgbe usoro njikọ a gasịrị, Fischer nakweere njikọ Koenigs-Knorr maka nyocha ya. N'iji usoro a, E.Fischer na B.Helferich bụ ndị mbụ t na-akọ njikọ nke alkyl glucoside ogologo agbụ na-egosipụta ihe ndị na-emepụta ihe na 1911.
N'ihe dị ka 1893, Fischer achọpụtala ihe ndị dị mkpa nke alkyl glycosides, dị ka nkwụsi ike ha dị elu na oxidation na hydrolysis, karịsịa na mgbasa ozi alkaline siri ike. Njirimara abụọ a bara uru maka alkyl polyglycosides na ngwa surfactant.
Nnyocha metụtara mmeghachi omume glycosidation ka na-aga n'ihu na ọtụtụ ụzọ na-adọrọ mmasị na glycosides ka emepụtara n'oge gara aga. A chịkọtara ụfọdụ usoro maka njikọ nke glycosides na eserese 5.
Na mkpokọta, usoro glycosidation kemịkalụ nwere ike kewaa n'ime usoro na-eduga n'usoro oligomer equilibria dị mgbagwoju anya na mgbanwe glycosyl acid-catalysed.
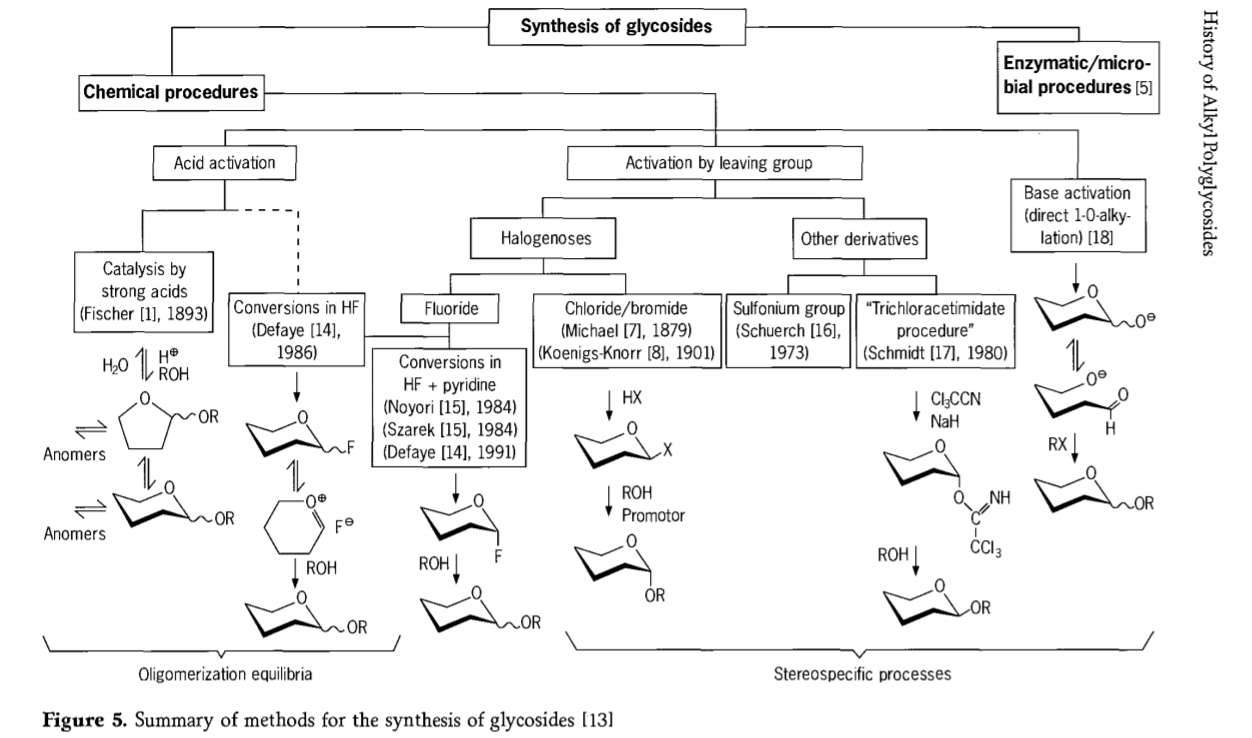
Mmeghachi omume na substrates carbohydrate arụ ọrụ nke ọma (mmeghachi omume Fischer glycosidic na mmeghachi omume hydrogen fluoride (HF) na ụmụ irighiri carbohydrate na-echebeghị) yana njikwa kinetics, enweghị mgbagha, yana ọkachasị mmeghachi omume nnọchi stereotaxic. Ụdị usoro nke abụọ nwere ike iduga n'ichepụta ụdị mmadụ n'otu n'otu kama na ngwakọta mgbagwoju anya nke mmeghachi omume, karịsịa mgbe ejikọtara ya na usoro nchekwa otu. Carbohydrates nwere ike ịhapụ otu dị na carbon ectopic, dị ka halogen atọm, sulfonyls, ma ọ bụ otu trichloroacetimidate, ma ọ bụ mee ka ntọala rụọ ọrụ tupu agbanwee na triflate esters.
N'ihe gbasara glycosidations na hydrogen fluoride ma ọ bụ ngwakọta nke hydrogen fluoride na pyridine (pyridinium poly [hydrogen fluoride]), glycosyl fluorides na-etolite na ọnọdụ ma na-agbanwe ya nke ọma na glycosides, dịka ọmụmaatụ na mmanya. E gosiputara fluoride hydrogen ka ọ bụrụ usoro mmeghachi omume na-arụ ọrụ siri ike, nke na-adịghị emebi emebi; Equilibrium auto condensation (oligomerization) ka a na-ahụ dị ka usoro Fischer, n'agbanyeghị na usoro mmeghachi omume nwere ike ịdị iche.
Kemịkalụ dị ọcha alkyl glycosides dabara naanị maka ngwa pụrụ iche. Dịka ọmụmaatụ, ejirila alkyl glycosides mee ihe nke ọma na nyocha biochemical maka kristal proteins membrane, dị ka kristal akụkụ atọ nke porin na bacteriorhodopsin n'ihu octyl β-D-glucopyranoside (nnwale ndị ọzọ dabere na ọrụ a na-eduga na Nrite Nobel na kemịkalụ maka Deisenhofer, Huber na 88 Michel).
N'oge mmepe nke alkyl polyglycosides, a na-eji usoro stereoselective mee ihe n'ọtụtụ nyocha iji mepụta ụdị ihe atụ dị iche iche na iji mụọ ihe onwunwe physicochemical ha, n'ihi mgbagwoju anya ha, enweghị ike nke etiti na ọnụọgụ na ọdịdị dị egwu nke ndị na-emebi usoro, nchịkọta nke ụdị Koenigs-Knorr na nsogbu ndị ọzọ na-echebe akụ na ụba ga-emepụta. Usoro ụdị Fischer na-adịchaghị mgbagwoju anya ma dị mfe ime n'ọ̀tụ̀tụ̀ azụmaahịa ma n'ihi ya, bụ usoro a na-ahọrọ maka mmepụta nke alkyl polyglycosides n'ọtụtụ buru ibu.
Oge nzipu: Sep-12-2020





